5 Key FAQs about Independent Drug Monographs
Do the clinicians in your practice know about the independent drug monographs in MedicalDirector’s Clinical software? In this article we take a look at 5 key FAQs about independent drug monographs and uncover why they’re such a valuable resource for busy healthcare professionals.
1. What is an Independent Drug Monograph?
Drug monographs contain similar clinical information to proprietary Product Information leaflets (PIs), but also include other information sourced from Australian and international medical literature.
Independent Drug Monographs are authored and carefully curated by the Editorial team within the Clinical Content division of MedicalDirector. The monographs are evidence-based and draw upon an extensive range of Australian and international sources. Written by a team of registered and practicing pharmacists, their creation follows a rigorous editorial process, with oversight and guidance provided by an Editorial Advisory Committee.
The Independent Drug Monographs in MedicalDirector Clinical are unique to MedicalDirector, and give you reassurance that you’re using current and accurate information. Other products rely solely on proprietary pharmaceutical company product information documents (PIs) as a source of medicines information. This can be risky as not all product information documents are current, and there are many examples of this in the Australian medical literature.*
2. How many Independent Drug Monographs are there in MedicalDirector Clinical?
There are over 600 Independent Drug Monographs in MedicalDirector Clinical. These same drug monographs are also used by universities, state health departments, hospital and retail pharmacies across Australia. This means you have access to the same reference material as all these other clinicians in your care network.
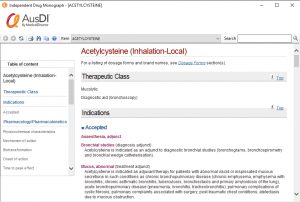
3. How do I access Independent Drug Monograph information?
To access Independent Drug Monograph information within your MedicalDirector Clinical software, simply follow these steps:
- Click the

- Right-click a patient’s current prescription, and select Drug Monograph from the menu,
- Right-click a medication from the Select Drug window (accessible whilst creating a new prescription) and select Drug Monograph from the menu.
4. How do I use the monograph?
Some of the useful information contained in the monographs include:
- Full-text, evidence-based drug, drug-class and complementary medicines monographs developed independently of pharmaceutical company influence
- Patient counseling points
- Patient monitoring recommendations
- Laboratory value alterations
- Precautions for geriatric, pregnant and paediatric patients
- Common, less common and rare adverse effects
- Off-label uses (where relevant)
These can be easily navigated from the table of contents on the left hand side of the Independent Drug Monograph screen, click the 
5. How do I found out more?
Click here to find out more about how to get the most value from your Independent Drug Monographs in your MedicalDirector Clinical Software.
*[MJA 2009 190 (3) 110–111]
AusDI is Australia’s trusted medicines information database. To find out more, visit ausdi.com.